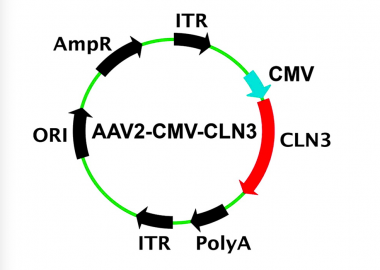
A recent WIVR study demonstrating the development of a gene therapy vector for treatment of CLN3 Batten disease was circulated as an article of interest by the journal, Human Gene Therapy (LINK). The article entitled “Using Patient-Specific Induced Pluripotent Stem Cells and Wild-type Mice to Develop a Gene Augmentation-based Strategy to Treat CLN3-associated Retinal Degeneration” summarized the results from a study of induced pluripotent stem cells generated from two patients with molecularly confirmed CLN3-associated Batten disease. A clinical grade gene-therapy carrying the full-length coding sequenc of human CLN3 was generated in our FDA-registered cGMP facility (the Dezii facility). The therapeutic was found to restore the CLN3 transcript and protein, and with no evidence of retinal toxicity in a safety study mice. This study demonstrates proof-of-principle, and serves as the foundation for initiating a clinical trial using the same strategy for the treatment of children with CLN3-associated retinal degeneration.
